

Jiang Changtao
Professor

Professor Jiang Changtao is a Full Professor and Boya Distinguished Professor at the Peking University School of Basic Medical Sciences, the Deputy Dean at School of Basic Medical Sciences, and a doctoral supervisor. He received the National Science Fund for Distinguished Young Scholars and the Xplorer Prize.
Department of Physiology and Pathophysiology
Research Interest: The gut microbes and the pathogenesis of metabolic diseases
Email: jiangchangtao@bjmu.edu.cn
Brief Introduction:
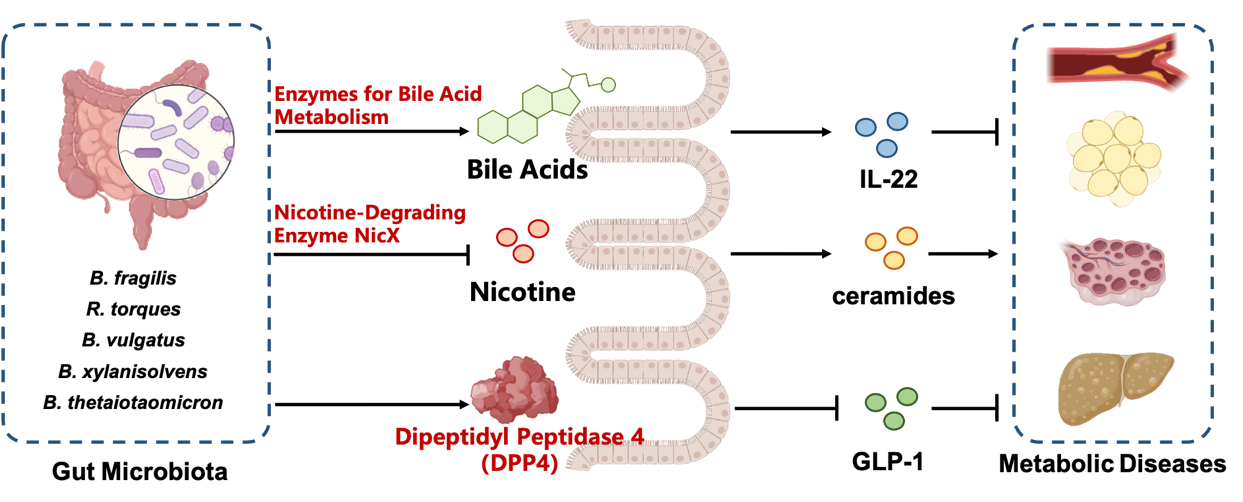
Prof. Jiang Changtao proposed a new theory of "Intestine as the Target in Treating Metabolic Diseases". He presented for the first time the concept of gut Microbial-Host isozymes (MHIs), identified the bacterial-derived isozyme dipeptidyl peptidase 4 (DPP4) and highlighted its important role in metabolic diseases (Science, 2023). He identified an endogenous nicotine-degrading gut microbiota and proposed a novel intervention strategy for gut bacteria to alleviate metabolic diseases through the nicotine-degrading enzyme NicX (Nature, 2022). He revealed the new paradigm of host reverse regulation of gut microbial metabolism. It was found that host intestinal HIF-2α remodeled the enzyme profile of bacterial bile acid metabolism and induced metabolic diseases (Cell Metabolism, 2021). He described the characteristic map of gut microbiota and their metabolites regulating metabolic diseases. He revealed that gut microbial metabolites bile acids, nicotine, ceramides and microbial-host isozyme DPP4 are the key mediators mediating the interaction between organs, and are the common pathogenesis of metabolic diseases. New targets for the intervention of metabolic diseases such as bacteria-derived DPP4, intestinal SMPD3, FXR, TGR5 and HIF-2α were discovered, and a series of biologic agents and drug candidates for the treatment of metabolic diseases such as Dau-d4, the gut bacterium Bacteroides xylanisolvens, bile acids GUDCA and HCA, IL-22 and PT2385 were developed.
In the past 5 years, Prof. Jiang Changtao has published more than 20 SCI papers in Science, Nature, Nature Medicine (3 articles), Cell Metabolism (3 articles) and others as the corresponding author. Among them, three articles were selected as highly cited papers, one article received F1000Prime recommendation with the highest rating score, and eight articles gained the recommendations from internationally renowned scholars and contemporaneous reviews. He was invited to publish five reviews in Nature Reviews Endocrinology, Cell Host & Microbe and other journals and holds 7 patents. He received the Xplorer Prize, the China Youth Science and Technology Award, the Shulan Medical Youth Award, the Chinese-American Diabetes Association (CADA) Young Investigator Award, the Mao Yisheng Beijing Youth Science and Technology Award and other awards. He is in charge of the projects supported by the Key Program of the National Natural Science Foundation of China, the Major Research Plan of the National Natural Science Foundation of China and the National Key Research and Development Program of China. He is the principal investigator for a project supported by the Science Fund for Creative Research Groups of the National Natural Science Foundation of China.
Summary of the achievements of the new theory of "Intestine as the Target in Treating Metabolic Diseases"
Publications:
1) Wang K#, Zhang Z#, Hang J#, Liu J#, Guo F#, Ding Y, Li M, Nie Q, Lin J, Zhuo Y, Sun L, Luo X, Zhong Q, Ye C, Yun C, Zhang Y, Wang J, Bao R, Pang Y, Wang G*, Gonzalez FJ*, Lei X*, Qiao J*, Jiang CT*. Microbial-host-isozyme analyses reveal microbial DPP4 as a potential antidiabetic target. Science. 2023 Aug 4;381(6657).
2) Chen B#, Sun L#, Zeng G#, Shen Z#, Wang K#, Yin L#, Xu F, Wang P, Ding Y, Nie Q, Wu Q, Zhang Z, Xia J, Lin J, Luo Y, Cai J, Krausz K, Zheng R, Xue Y, Zheng MH*, Li Y*, Yu C*, Gonzalez FJ*, Jiang CT*. Gut bacteria alleviate smoking-related NASH by degrading gut nicotine. Nature. 2022 Oct;610(7932):562-568. (Commentaries and editorials from Nat Metab. 2022 Nov, Nat Rev Microbiol. 2022 Nov)
3) Wu Q#, Liang X#, Wang K#, Lin J, Wang X, Wang P, Zhang Y, Nie Q, Liu H, Zhang Z, Liu J, Pang Y, Jiang CT*. Intestinal hypoxia-inducible factor 2α regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab. 2021 Oct 5;33(10):1988-2003.
4) Sun L#, Xie C#, Wang G#, Wu Y#, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, Zhang S, Yun C, Lian G, Zhang X, Zhang H, Bisson WH, Shi J, Gao X, Ge P, Liu C, Krausz KW, Nichols RG, Cai J, Rimal B, Patterson AD, Wang X, Gonzalez FJ, Jiang CT*. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018 Dec;24(12):1919-1929. (Highly cited paper, data from Essential Science Indicators; Commentaries and editorials from Nat Med. 2018, Cell Metab. 2018, Nat Rev Endocrinol. 2018)
5) Zhang X#, Zhang Y#, Wang P#, Zhang SY#, Dong Y, Zeng G, Yan Y, Sun L, Wu Q, Liu H, Liu B, Kong W, Wang X, Jiang CT*. Adipocyte hypoxia-inducible factor 2α suppresses atherosclerosis by promoting adipose ceramide catabolism. Cell Metab. 2019 Nov 5;30(5):937-951.
6) Wu Q#, Sun L#, Hu X#, Wang X, Xu F, Chen B, Liang X, Xia J, Wang P, Aibara D, Zhao S, Zeng G, Yun C, Yan Y, Zhu Y, Bustin M, Zhang SY*, Gonzalez FJ*, Jiang CT*. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J Clin Invest. 2021 May 3;131(9):e142865.
7) Zheng X#, Chen T#, Jiang R#, Zhao A#, Wu Q, Kuang J, Sun D, Ren Z, Li M, Zhao M, Wang S, Bao Y, Li H, Hu C, Dong B, Li D, Wu J, Xia J, Wang X, Lan K, Rajani C, Xie G, Lu A, Jia WP*, Jiang CT*, Jia W*. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021 Apr 6;33(4):791-803.
8) Qi X#, Yun C#, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Wang L, Gonzalez FJ, Patterson AD, Liu H, Mu L, Zhou Z, Zhao Y, Li R, Liu P, Zhong C, Pang Y*, Jiang CT*, Qiao J*. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019 Aug;25(8):1225-1233. (Commentaries and editorials from Nat Med. 2019, Cell Metab. 2019, Nat Rev Drug Discov. 2019)
9) Xie C, Yagai T, Luo Y, Liang X, Chen T, Wang Q, Sun D, Zhao J, Ramakrishnan SK, Sun L, Jiang C, Xue X, Tian Y, Krausz KW, Patterson AD, Shah YM, Wu Y*, Jiang CT*, Gonzalez FJ*. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat Med. 2017 Nov;23(11):1298-1308. (Commentaries and editorials from Nat Med. 2017, Nat Rev Gastroenterol Hepatol. 2017)
10) Li X#, Zhang X#, Xia J#, Zhang L, Chen B, Lian G, Yun C, Yang J, Yan Y, Wang P, Wang X, Liu B, Liu H, Liang H, Pang Y, Wang X*, Jiang CT*. Macrophage HIF-2α suppresses NLRP3 inflammasome activation and alleviates insulin resistance. Cell Rep. 2021 Aug 24;36(8):109607.

Group Photo
Bakup

His long-term goal is to elucidate the underlying pathways of inter-organ crosstalk and to define how this crosstalk that in cooperation with diet and metabolism by gut microbiota to affect metabolic diseases, such as obesity, insulin resistance, nonalcoholic fatty liver disease (NAFLD), atherosclerosis, and polycystic ovary syndrome (PCOS). His major contributions are: 1) Revealing that intestine-derived ceramides were regulated by the transcription factors farnesoid X receptor (FXR) and hypoxia-inducible factor 2α (HIF-2α) to potentiate metabolic diseases. And identified that intestinal FXR and HIF-2α are viable targets for the treatment of metabolic diseases. He discovered a human endogenous FXR antagonist, glycoursodeoxycholic acid (GUDCA), and developed a highly specific FXR antagonists, glycine-muricholic acid (Gly-MCA) as well as its derivatives, as potential drugs to treat obesity, insulin resistance and NAFLD. Furthermore, his study elucidated that a pathway involving the gut microbiota-bile acid signaling axis that presents a novel possible treatment strategy for PCOS-related insulin resistance, ovarian dysfunction and infertility. He proposed that changes in gut bacteria, intestinal microenvironment and bile acids metabolism pathways regulate metabolic diseases, which has led to the discovery of novel drug targets ; 2) Elucidating the opposing functions of adipose HIF-1α and HIF-2α in the regulation of ceramide metabolism. Notably, he identified the ceramide-synthesis gene encoding sphingomyelin phosphodiesterase 3 (SMPD3) and ceramide-degradation gene encoding alkaline ceramidase 2 (ACER2) as novel target genes of HIF-1α and HIF-2α, respectively. His group demonstrated that adipocyte HIF-1α and HIF-2α are thermogenic regulators and further revealed the inverse effects of adipose HIF-1α and HIF-2α on the development of atherosclerosis and NAFLD by controlling ceramide metabolism.

